AGAcell® is an investigational pre-clinical cell-therapy program that explores the capacity to restore the cellular niche of the hair follicle, to promote long-term hair growth through a minimally invasive, non-surgical procedure. It is the first preclinical program based on an allogeneic therapy from isolated and expanded mesenchymal stem cells specifically for the treatment of androgenetic alopecia.

Androgenetic alopecia (AGA) is a condition that leads to progressive hair loss in millions of men and women worldwide. As one of the most common causes of hair loss, it represents one of the largest segments in the global hair health market.
There are mainly two ways of treating androgenetic alopecia: pharmacological treatments and surgical hair transplants. Below is an overview of the most important significant therapeutic options, and highlights key considerations that might limit their effectiveness and overall use.
Effects are temporary and limited
Chronic use can cause significant side effects
Cannot be performed on all patients
High cost
Adyuvant therapies are required
Feasibility and success depends on patient scalp characteristics and AGA progression
No clinical evidence available
Frequent use is required for effective outcomes
Medium-high cost

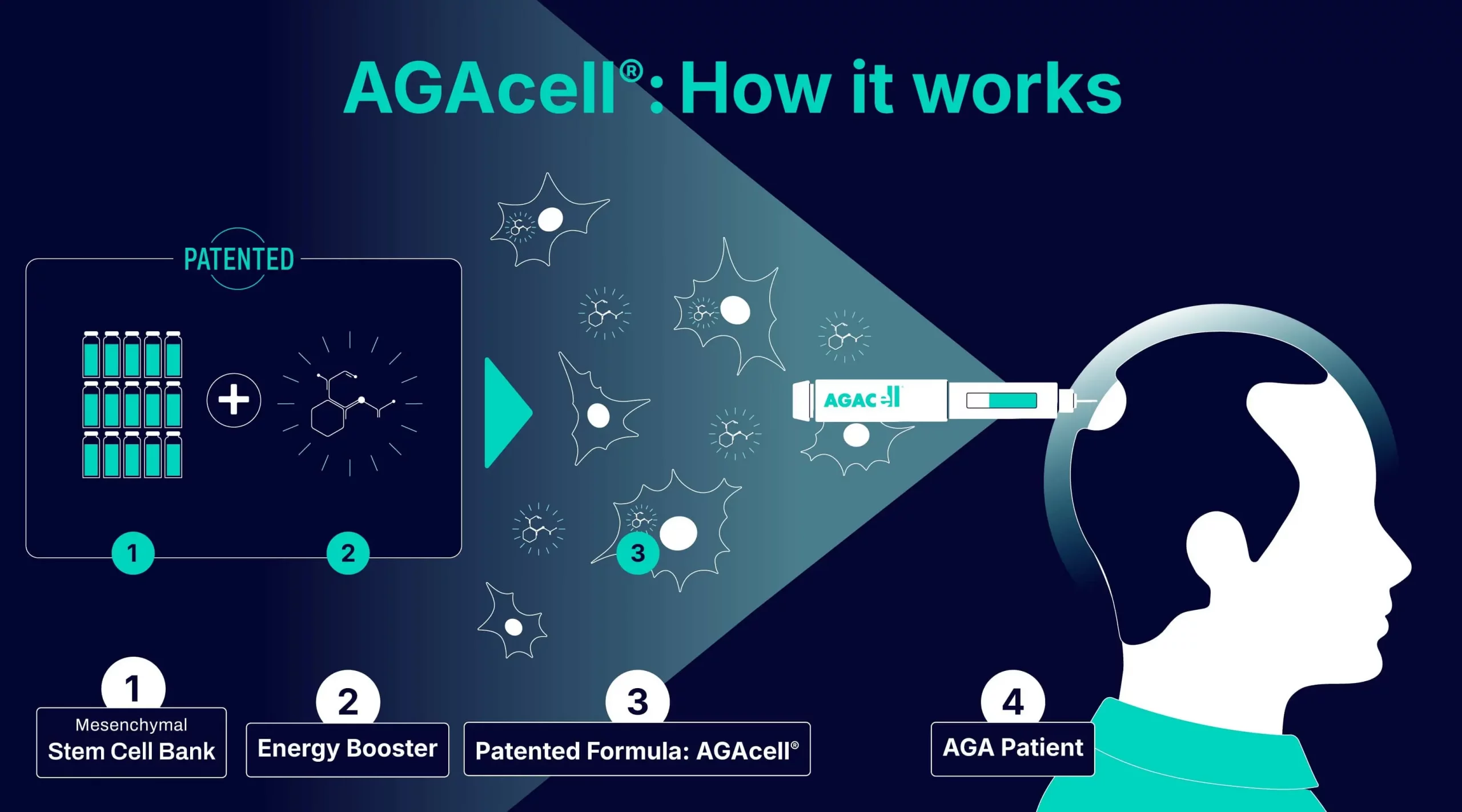
AGAcell® is a patented formulation that combines allogeneic mesenchymal stem cells with a bioactive molecule intended to enhance their regenerative potential. Current pre-clinical studies are examining whether this synergy can modulate the follicular micro-environment and help counteract damage associated with hair-loss progression. Exploratory data in animal models have yielded encouraging results, supporting its further evaluation in human trials.
“AGAcell® is in the pre-clinical stage with ongoing development toward future clinical application.”
Our GMP compliant manufacturing process starts with the screening of a healthy donor, from whom we extract and isolate mesenchymal stem cells. Subsequently, we perform cell expansion under rigorously controlled conditions to achieve an optimal quantity of viable cells which are then cryopreserve in a mesenchymal stem cell bank.
Mesenchymal stem cells (MSCs) are being investigated for their capacity to interact with the resident follicular cells and support regeneration of the hair-follicle niche. AGAcell® combines these allogeneic MSCs with a bioactive molecule that synergistically enhances their regenerative activity.
The clinical planned procedure involves intradermal administration into the affected scalp area by a qualified dermatologist. Ongoing studies aim to determine whether this minimally invasive approach can modulate the follicle’s cellular niche, restoring hair-growth potential and long-lasting solution for AGA.
As a potential allogeneic therapy, AGAcell® is based on mesenchymal stem cells sourced from a qualified donor and stored in a cell bank. Unlike autologous procedures, where cells are harvested from, and later re-administered to, the same patient, an allogeneic strategy may offer several practical advantages:

Allogeneic MSCs are harvested from rigorously screened donor tissue and expanded under standardized GMP conditions, yielding more consistent viability and potency than autologous cells, whose quality can vary with each patient’s age, comorbidities and disease state.
Cells from a single donor batch can be expanded under GMP conditions for multiple doses, which could reduce per-patient manufacturing costs compared with one-patient-one-batch autologous processes.
The envisaged protocol requires only a single visit for intradermal administration; autologous approaches usually need a first visit for cell harvest and a second for reinfusion. Recovery is much faster and less painful compared to surgical hair transplants.
As a cryopreserved, “ready-to-use” product, treatment could in principle be scheduled soon after prescription, without the lead time needed to culture each patient’s own cells.
AGAcell® is planned to be manufactured under GMP compliant conditions, meeting the same quality and safety requirements applied to autologous ATMPs.
















To provide the best experiences, we use technologies such as cookies to store and/or access device information. Consent to these technologies will allow us to process data such as browsing behavior or unique identifiers on this site. Not consenting or withdrawing consent may negatively affect certain features and functions.